Warning: Not All Cold Sore Medicines Are Created Equal
Survey Commissioned by Abreva Finds Majority of Americans Don’t Check Ingredients and Choose Medicines Based on Packaging
PARSIPPANY, NJ –Looks can be deceiving, especially with all the choices and sophisticated packaging that can be found in the OTC cold sore category. Comparing ingredients could make the difference between buying a cold sore medicine that heals versus a product that simply does not heal cold sores. Abreva, a leading OTC cold sore treatment brand, recently commissioned a study to draw attention to the importance of comparing the ingredients in branded versus ‘look-alike’ products, and the findings are surprising.
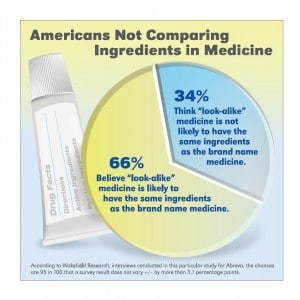
In the survey, which asked 1,000 American adults, ages 18 and older about how closely they pay attention to the ingredients in “look-alike” treatments, it revealed 66 percent of Americans believe that when “look-alike treatments” have the same or similar packaging as the name-brand treatment, that it is likely that they have the same ingredients. Additionally, 93 percent of Americans admit to purchasing the “look-alike treatments” in the past solely because they are less expensive.
“All of these “look-alike” cold sore treatments have different ingredients from the Abreva brand they are trying to imitate,” said, Pam Marquess, Pharm.D, pharmacist and pharmacy chain owner and GSK spokesperson. “Even though two items on the shelf may have claims and packaging similar to Abreva, the inside is a different story. Comparing ingredients is critical to selecting a proper cold sore treatment.”
To treat cold sores, there is only one over-the-counter product available that is FDA approved to speed the healing of cold sores. That product is Abreva, which contains 10 percent docosanol. Several “look-alike” cold sore treatments tout healing claims, but contain the ingredient Benzalkonium Chloride instead of docosonal. The FDA recently issued a warning letter to a marketer and distributor of a product containing benzalkonium chloride that is making the claim on its product’s label that it heals cold sores. The FDA found that the active ingredient, benzalkonium chloride, is not indicated as a cold sore treatment and may not make cold sore healing claims because there is no scientific evidence to support claims that it heals cold sores.
“Without comparing labels and ingredients, you may think the “look-alike” treatment will heal your cold sore just like Abreva, but benzalkonium chloride does not heal cold sores,” said Marquess.
“Hopefully the findings of this survey will compel people looking for a cold sore healing treatment to compare the ingredients between Abreva and ‘look-alike’ products for their own protection,” said Lisa Maxwell-Anekwe, senior brand manager, Abreva. “For the safe and effective treatment of cold sores, it’s important to take those few extra seconds and compare more than price, but compare ingredients. Make sure you are spending your hard-earned money on what really works.”
“If you’re in doubt about what cold sore treatment to look for, ask your pharmacist if both Abreva and “look-alike” products have the same active ingredients. This will allow you to make an educated purchasing decision based on facts and not packaging or cost,” said Marquess. “If you want to heal your cold sore fast, it’s better to spend more on something that really works than spend a little on something that doesn’t. The other great thing about Abreva is that it also comes with a 100 percent satisfaction guarantee.” For more information visit Abreva vs. the others on www.Abreva.com
About GlaxoSmithKline Consumer Healthcare
GlaxoSmithKline Consumer Healthcare is one of the world's largest over-the-counter consumer healthcare products companies. Its well-known brands include Nicorette® and NicoDerm® CQ, the leading smoking cessation products; abreva, the only FDA-approved OTC cold sore treatment to speed healing; alli, the only FDA-OTC weight loss aid; as well as medicine cabinet staples, Aquafresh®, Sensodyne,® Tums® and Breathe Right,® all of which are trademarks owned by and/or licensed to GlaxoSmithKline Group of Companies.
Methodological Notes
The Abreva Survey was conducted by Wakefield Research (www.wakefieldresearch.com) among 1,000 American adults, ages 18 and older, between April 26th and May 2nd, 2012, using an email invitation and an online survey. Quotas have been set to ensure reliable and accurate representation of the U.S. adult population 18 and older.
Results of any sample are subject to sampling variation. The magnitude of the variation is measurable and is affected by the number of interviews and the level of the percentages expressing the results. For the interviews conducted in this particular study, the chances are 95 in 100 that a survey result does not vary, plus or minus, by more than 3.1 percentage points from the result that would be obtained if interviews had been conducted with all persons in the universe represented by the sample.
